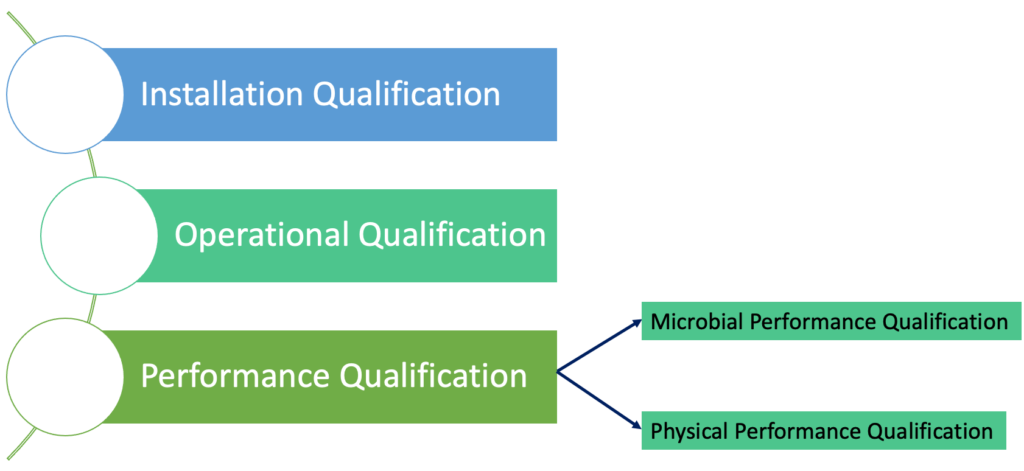
Medical device software and sterilization professionals - We are proud to announce the CPD accreditation of our online course regarding validation of sterilization according to EN ISO 11135. This is important for

DIN EN ISO 11135:2014 - Sterilization of health care products - Ethylene oxide - Requirements for the development, validation and routine control of a sterilization process for medical devices (ISO 11135:2014); German version EN ISO 11135:2014

ISO 11135 2014 Certification Service in New Delhi, QACS International Private Limited | ID: 26172685397

ANSI/AAMI/ISO 11135:1994 - Medical devices-Validation and routine control of ethylene oxide sterilization, 3ed

BS EN ISO 11135-1:2007 - Sterilization of health care products. Ethylene oxide. Requirements for development, validation and routine control of a sterilization process for medical devices (British Standard)

ISO 11135:2014 - Sterilization of health-care products — Ethylene oxide — Requirements for the development, validation and routine control of a sterilization process for medical devices

SGS Academy United Kingdom - Introduction To Ethylene Oxide (EO) Sterilisation and ISO 11135 (On-Site)
UNE EN ISO 11135:2015/A1:2020 Sterilization of health-care products - Ethylene oxide - Requirements for